Drug Label Safety Checker
Verify you've reviewed these critical sections from your FDA drug label:
Review your label sections below to ensure medication safety. This tool helps you verify critical information from FDA drug labels.
When you pick up a prescription, the tiny paper insert in the box isn’t just filler. It’s a legally required, highly detailed document created by the FDA to keep you safe. But most people never read it-because it looks like a textbook written in legalese. The truth? If you know where to look, those labels hold critical answers about side effects, dosing, and risks you won’t find on the pharmacy screen or even in your doctor’s quick explanation.
What You’re Actually Looking At: The FDA’s Official Drug Label
The document you’re holding is called the United States Prescribing Information (USPI). It’s not a marketing brochure. It’s a regulatory requirement. Every prescription drug sold in the U.S. must follow this exact format, standardized since 2006 under the Physician Labeling Rule. The FDA didn’t do this to make life harder for doctors-they did it because, before 2006, medication errors were common. A 2020 analysis from the NCBI Bookshelf found that over half of dosing mistakes happened because the label was unclear or disorganized.
The label has three main parts: the Highlights, the Table of Contents, and the Full Prescribing Information (FPI). The Highlights are just half a page. They’re meant to give you the fastest snapshot: what the drug treats, the most serious warnings, and the standard dose. But here’s the catch: the Highlights say right on them that they’re incomplete. You can’t rely on them alone. The real power is in the FPI-the 17 sections that follow.
Section 1: Indications and Usage
This is where you find out exactly what the drug is approved to treat. Not what your doctor thinks it might help with. Not what you read online. Only what the FDA has reviewed and approved based on clinical trials.
For example, if a drug says it’s for "type 2 diabetes in adults," that means it hasn’t been tested or approved for children, type 1 diabetes, or prediabetes. If your doctor prescribes it for something else-that’s called off-label use. It’s legal, but it’s not what the label says. Always check this section to make sure your condition matches the approved use.
Section 2: Dosage and Administration
This is the most misunderstood section. It doesn’t just say "take one pill daily." It breaks down dosing by age, weight, kidney function, liver problems, and even what time of day to take it. It also tells you what to do if you miss a dose, how to adjust for other medications, and whether food affects absorption.
Take a common blood thinner like rivaroxaban. The label might say: "For atrial fibrillation: 20 mg once daily with the evening meal. For patients with moderate renal impairment: reduce to 15 mg daily." If you have kidney disease and don’t adjust the dose, you could bleed internally. That’s why this section isn’t optional. It’s life-or-death math.
Section 5: Warnings and Precautions (Including the Boxed Warning)
This is the most important section for safety. And it starts with the Boxed Warning-a thick black border at the top of the FPI. It’s the FDA’s highest alert. If a drug has one, it means there’s a risk of death, severe injury, or permanent damage if used incorrectly.
For example, fluoxetine (Prozac) has a Boxed Warning for increased suicidal thoughts in children and young adults under 25. Opioids have Boxed Warnings for addiction, respiratory depression, and accidental overdose. If you see this box, read the entire section. It explains exactly who’s at risk, what symptoms to watch for, and what to do if they appear.
Section 6: Adverse Reactions
This section lists side effects-but not just any side effects. Only those seen in clinical trials, grouped by how often they happened. "Very common" means more than 1 in 10 people had it. "Common" is 1 in 10 to 1 in 100. "Rare" is less than 1 in 1,000.
Many people panic when they see a list of 50 side effects. But the truth? Most people get none or just one or two mild ones. The key is to look for the ones that are serious and common. For example, if a statin lists "muscle pain" as common and "rhabdomyolysis" (a dangerous muscle breakdown) as rare, you know muscle soreness is likely harmless, but unexplained weakness or dark urine needs immediate attention.
Section 7: Drug Interactions
This section tells you what other drugs, foods, or supplements could make this medication dangerous or useless. It’s not just about other pills. It includes grapefruit juice, St. John’s wort, alcohol, and even some antacids.
Take warfarin (Coumadin). The label will warn you that antibiotics like clarithromycin can make it too strong and cause bleeding. Or that vitamin K-rich foods (like kale or spinach) can make it less effective. If you’re on multiple medications, this section is your safety net. Always check it before starting a new drug-even an over-the-counter one.
Section 8: Use in Specific Populations
Is the drug safe during pregnancy? While breastfeeding? For seniors? For kids? This section answers those questions with data, not guesses.
Many drugs are not tested in pregnant women, so the label might say "Use only if potential benefit justifies potential risk to fetus." That’s not a green light-it’s a red flag. For older adults, the label might say "Dose reduction recommended due to decreased kidney function." That’s why seniors often need lower doses, even if the label says "adult dose." Don’t assume one-size-fits-all.
Section 16: How Supplied/Storage and Handling
This is where you find the National Drug Code (NDC)-a 10-digit number that uniquely identifies the drug, manufacturer, strength, and packaging. It’s used by pharmacies and insurers to track exactly what you got.
But more importantly, this section tells you how to store the drug. Some need refrigeration. Some must be kept away from light. Some expire quickly after opening. If you store it wrong, it can lose effectiveness or become unsafe. And if you see a different NDC than what’s on your old bottle? That could mean a generic switch or a formulation change. Always double-check.
Section 17: Patient Counseling Information
This is the section most doctors skip-but it’s written for you. It gives exact phrases your pharmacist or provider should use when explaining the drug. Things like: "Take this on an empty stomach," "Do not crush the tablet," "Watch for signs of dizziness when standing up."
Studies show only about 38% of providers actually use this section during patient counseling. That means you might not get the full safety message. So read it yourself. If your doctor didn’t mention something listed here, ask why.
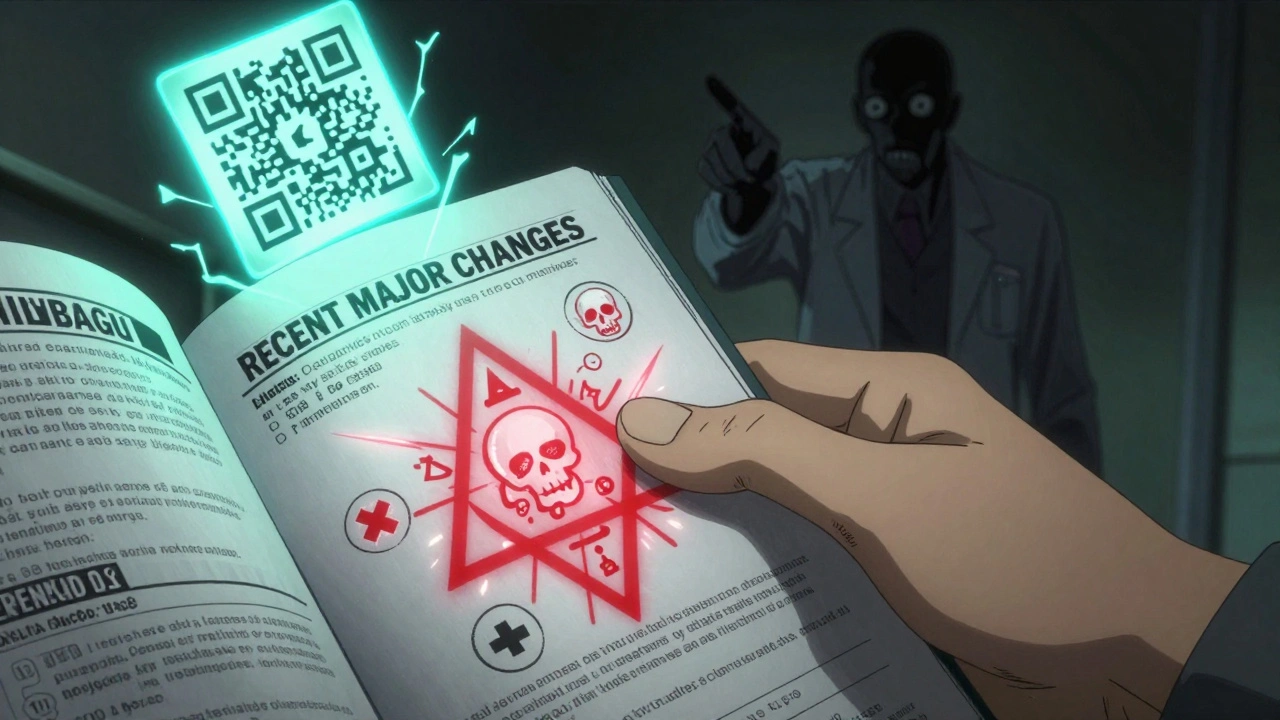
Recent Major Changes: Don’t Skip This
Since 2018, every label must include a "Recent Major Changes" section. It lists every section that was updated in the last six months. That’s critical. A drug might have had a new warning added last month about liver damage. If you’re still using an old label, you’re working with outdated info.
The FDA tracks that these updates reduce delays in safety awareness by 35%. So before you refill a prescription, glance at this section. If something changed, read the full update. It could mean avoiding a dangerous interaction or adjusting your dose.
How to Use This in Real Life
Here’s how to make this practical:
- When you get a new prescription, ask for the full prescribing information. Pharmacies can print it or email it.
- Open it to Section 5 first. Look for the Boxed Warning. If there’s one, read it all.
- Go to Section 2. Confirm the dose matches what your doctor told you.
- Check Section 7. Look for interactions with anything else you take-supplements included.
- Flip to Section 17. See what your provider should’ve told you. Ask if they did.
- Every time you refill, check the "Recent Major Changes" section.
It takes 5 minutes. But that 5 minutes can prevent a hospital visit.
Why This Matters More Than Ever
The FDA is moving toward digital, interactive labels. By 2027, many new drugs may come with apps or QR codes that let you drill down into information based on your age, kidney function, or other meds. But until then, the paper label is your only guaranteed source of truth.
And it’s not just for doctors. Patients who read their labels are 29% less likely to make medication errors, according to a 2023 Johns Hopkins study. That’s not a small number. That’s a life saved.
Don’t let the size or complexity scare you. You don’t need to memorize all 17 sections. Just learn where to find the big ones: Boxed Warning, Dosing, Interactions, and Recent Changes. That’s your safety toolkit.
Is the FDA drug label the same as the pharmacy label?
No. The pharmacy label is a simplified version meant for quick reference-usually just the name, dose, and instructions. The FDA drug label (Full Prescribing Information) is the complete, legally required document with all safety data, clinical evidence, and warnings. Always use the FDA label for full understanding.
Can I trust the Highlights section alone?
No. The Highlights are designed to be a quick snapshot, but they’re intentionally incomplete. The FDA requires them to state that they don’t contain all information. Relying only on Highlights can lead to missing critical warnings, dosing rules, or interactions. Always refer to the Full Prescribing Information for complete details.
What if my doctor prescribes a drug for something not listed in Section 1?
This is called off-label use and is legal. But it means the FDA hasn’t reviewed the drug for that specific condition. Ask your doctor why they’re prescribing it this way, what evidence supports it, and whether there are safer or approved alternatives. Don’t assume it’s safe just because your doctor recommends it.
How often do FDA drug labels change?
Labels are updated whenever new safety data emerges. On average, a drug’s label changes every 14.3 months. Some updates are minor; others add new Boxed Warnings or contraindications. Always check the "Recent Major Changes" section before refilling any prescription.
Are generic drugs’ labels the same as brand-name ones?
Yes. Generic drugs must have identical Active Ingredients, Strength, Dosage Form, Route of Administration, and labeling to the brand-name version. The only differences are in the inactive ingredients (like fillers) and packaging. The safety and usage info is exactly the same.
What should I do if I find an error on the FDA label?
If you spot a clear error-like a wrong dose or missing warning-contact the drug manufacturer directly and report it to the FDA’s MedWatch program. You can file a report online at fda.gov/medwatch. Even small errors can be life-threatening if repeated. Your report helps improve safety for everyone.


Cindy Lopez
December 3 2025Most people don’t read the label because it’s designed to intimidate, not inform. I’ve seen pharmacists hand out these 20-page PDFs like they’re holy scripture-and then act surprised when patients just take the pill and hope for the best.
It’s not the patient’s fault. It’s the system’s failure.